Abstract
Background: AlloBMT is the only curative therapy for many patients with acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS). With the significant improvement in non-relapse mortality, disease relapse remains the main cause of therapeutic failure. The presence of leukemia-specific somatic mutations provides an attractive strategy for molecular assessment of measurable residual disease (MRD). The level of MRD at the time of complete remission (CR) has been an important predictor of relapse in patients undergoing alloBMT. Widely utilized molecular MRD assays require invasive bone marrow (BM) aspiration to ensure an adequate amount of residual leukemia cells. Circulating tumor DNA (ctDNA) released from malignant cells can be reliably detected in patients' plasma and has been widely used in diagnosis, early detection, and disease burden monitoring in solid tumors. This assay is not widely used in myeloid malignancies as the proper assessment of its technical and clinical utility as an MRD monitoring strategy has not been performed. In the current study, we aimed to determine the differential sensitivity between bone marrow and ctDNA-based MRD and its utility as a minimally invasive diagnostic and disease monitoring tool. Additionally, we evaluated the clinical utility of dynamic ctDNA-based MRD monitoring and its effect on post-alloBMT relapse by using plasma samples from 2 large prospective clinical trials (BMT CTN 0201 and 0402).
Methods: The technical validation cohort included 20 consecutive patients with myeloid malignancies undergoing alloBMT at Johns Hopkins. A minimum of 10mL of plasma and bone marrow DNA was collected at the time of remission and at multiple time points after alloBMT.
The clinical validation cohort included 90 patients participating in two prospective alloBMT trials (BMT CTN-0201 and 0402). The eligibility criteria included the diagnosis of myeloid malignancy (72 AML, 18 MDS) and the minimum of 2mL of plasma available from day 0 and/or day 90 after alloBMT. Ultra-deep error-corrected targeted sequencing was performed on plasma and bone marrow-derived DNA. The variant allele frequency (VAF) per patient was represented by the maximum VAF of all detected mutations.
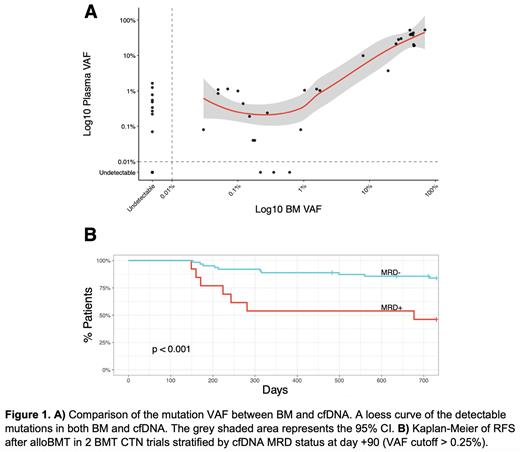
Results: To measure the exact contribution of hematopoietic tissue to plasma cell-free DNA (cfDNA) we analyzed cfDNA in alloBMT recipients. Using donor and recipient-specific polymorphisms we demonstrated that after full donor hematopoietic reconstitution by standard chimerism assays (day +180), 94.6% (range 93.9-95.3%) of cfDNA was of hematopoietic origin. To determine the differential sensitivity of the cfDNA vs. BM-based MRD we focused only on leukemia-specific mutations found in the diagnostic specimens. The minimum level of detection was 0.03%. Forty leukemia-specific mutations were detected in the technical validation cohort. Out of 31 mutations seen in the BM, only 3 were not detected in cfDNA (3/31, 9.7%). On the contrary, out of 37 mutations in cfDNA, 9 were not seen in BM (9/37, 25%). While we observed a strong positive correlation (rho = 0.8, p<0.0001) at any level of MRD between BM and cfDNA, cfDNA was superior at the lower MRD level (VAF cut-off < 0.26%).
The clinical utility of cfDNA MRD was utilized in a separate validation cohort of 90 patients enrolled on 2 BMT CTN trials. The median (Q1, Q3) follow-up was 26 months (25 - 27 months). cfDNA-MRD positivity (VAF cutoff >0.25%) at day +90 was associated with significantly worse relapse-free survival (RFS) (46.2% vs 84.0%, p<0.001) and overall survival (OS) at two years (53.8% vs 87.2%, p=0.0013) after alloBMT.
Conclusions: cfDNA-based MRD assessment appears to be a promising, minimally invasive test that provides important prognostic information in patients with myeloid malignancies undergoing alloBMT. The presence of cfDNA MRD at day 90 post alloBMT was associated with significantly worse RFS and OS in patients with myeloid malignancies. The dynamic assessment of MRD by cfDNA may not only provide important prognostic information but may also guide the therapeutic decision-making and timely implementation of post-alloBMT maintenance therapies to improve outcomes of patients with myeloid malignancies.
Disclosures
DeZern:CTI BioPharma: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; GERON: Other: DSMB; Syntrix Pharmaceuticals: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal